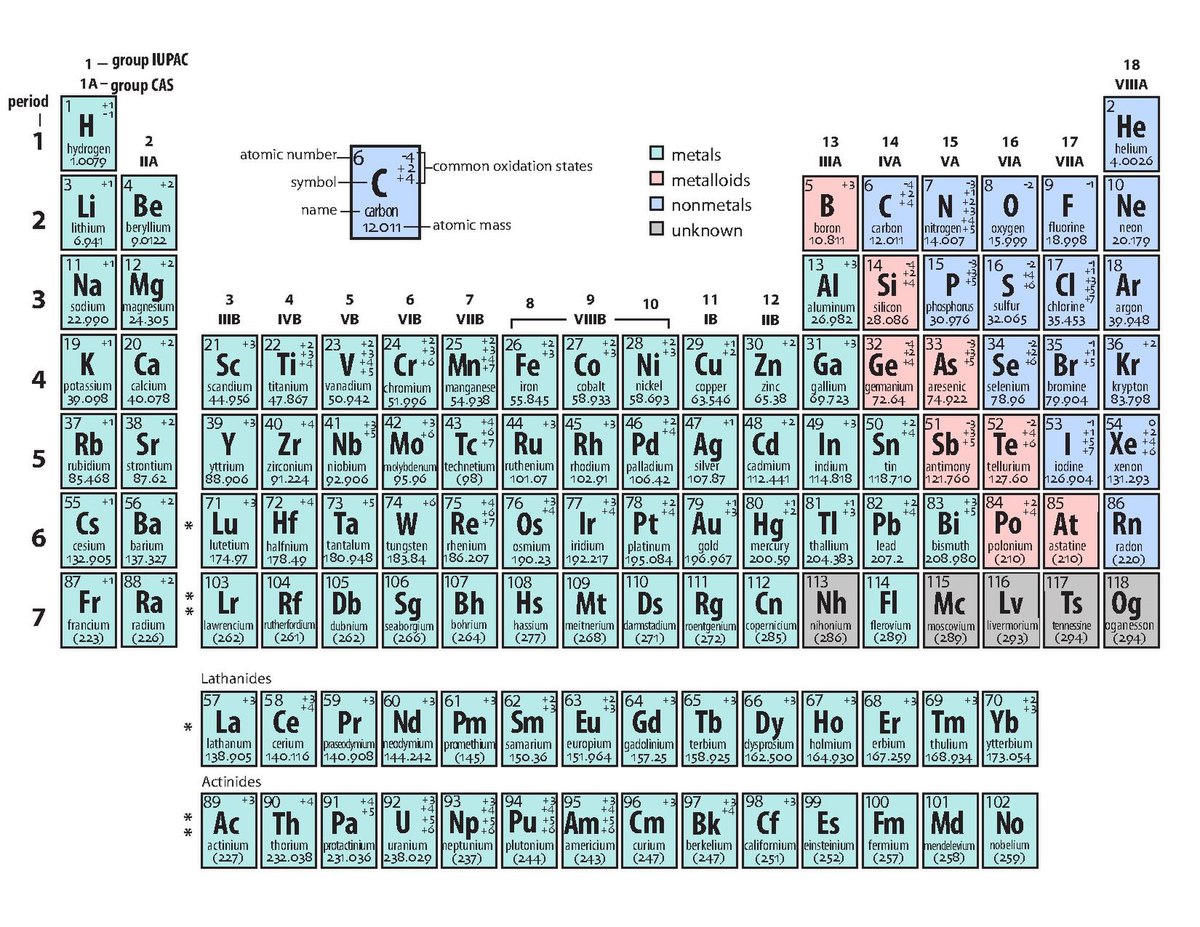
Alkali Metals |
Alkaline Earth Metals |
Transition Metals |
Post-tranistion Metals |
||||||||||||||||
| IA | Metalloids |
Non-Metals |
Noble Gases |
Lathanides |
Actinides |
VIIIA | |||||||||||||
|
1 H Hydrogen 1.0079 |
IIA | State | at | standard | temperature | and | pressure | IIIA | IVA | VA | VIA | VIIA |
2 He Helium 4.0026 |
||||||
|
3 Li Lithium 6.941 |
4 Be Beryllium 9.0122 |
Solid | Liquid | Gas |
5 B Boron 10.811 |
6 C carbon 12.011 |
7 N Nitrogen 14.007 |
8 O Oxygen 15.999 |
9 F Flourine 18.998 |
10 Ne Neon 20.179 |
|||||||||
|
11 Na Sodium 22.990 |
12 Mg Magnesium 24.305 |
IIIB | VIB | VB | VIB | VIIB | VIIIB | VIIIB | VIIIB | IB | IIB |
13 Al Aluminium 26.982 |
14 Si Silicon 28.086 |
15 P Phosphorus 30.976 |
16 S Sulphur 32.065 |
17 Cl Chlorine 35.453 |
18 Ar Argon 39.948 |
||
|
19 K Potassium 39.098 |
20 Ca Calcium 40.078 |
21 Sc Scandium 44.956 |
22 Ti Titanium 47.867 |
23 V Vanadium 50.942 |
24 Cr Chronium 51.996 |
25 Mn Manganese 54.938 |
26 Fe Iron 55.845 |
27 Co Cobalt 58.933 |
28 Ni Nickel 58.693 |
29 Cu Copper 63.546 |
30 Zn Zinc 65.38 |
31 Ga Gallium 69.723 |
32 Ge Germanium 72.64 |
33 As Aresenic 74.922 |
34 Se Selenium 78.96 |
35 Br Bromine 79.904 |
36 Kr Krypton 83.798 |
||
|
37 Rb Rubidium 85.468 |
38 Sr Strontium 87.62 |
39 Y Yttrium 88.906 |
40 Zr Zirconium 91.224 |
41 Nb Niobium 92.906 |
42 Mo Molybdenum 95.96 |
43 Tc Technetium 98 |
44 Ru Ruthenium 101.07 |
45 Rh Rhodium 102.91 |
46 Pd Palladium 106.42 |
47 Ag Silver 107.87 |
48 Cd Cadmium 112.441 |
49 In Indium 114.818 |
50 Sn tin 118.710 |
51 Sb Antimony 121.760 |
52 Te Tellurium 127.60 |
53 I Iodine 126.904 |
54 Xe Xenon 131.293 |
||
|
55 Cs Caesium 132.905 |
56 Ba Barium 137.327 |
* | lanthanides 57 - 71 |
72 Hf Halfnium 178.49 |
73 Ta Tantalum 180.948 |
74 W Tungsten 183.84 |
75 Re Rhenium 186.207 |
76 Os Osmium 190.23 |
77 Ir Iridium 192.217 |
78 Pt Platinum 195.084 |
79 Au Gold 196.967 |
80 Hg Mercury 200.59 |
81 Tl Thallium 204.383 |
82 Pb Lead 207.2 |
83 Bi Bismuth 208.980 |
84 Po Polonium 210 |
85 At Astatine 210 |
86 Rn Radon 220 |
|
|
87 Fr Francium 223 |
88 Ra Radium 226 |
** | Acticides 89 - 103 |
104 Rf Rutherfordium 261 |
105 Db Dubnium 262 |
106 Sg Seaborgium 266 |
107 Bh Bohrium 264 |
108 Hs Hassium 277 |
109 Mt Meitnerium 268 |
110 Ds Darmstadium 271 |
111 Rg Roentgenium 272 |
112 Cn Copernicium 285 |
113 Nh Nihonium 286 |
114 Fl Flerovium 289 |
115 Mc Moscovium 289 |
116 Lv Livermorium 293 |
117 Ts Tennessine 294 |
118 Og Oganesson 294 |
| * |
57 La lathanum 138.908 |
58 Ce Cerium 140.116 |
59 Pr Praseodymium 140.908 |
60 Nd Neodymium 144.242 |
61 Pm Promethium 145 |
62 Sm Samarium 150.36 |
63 Eu Europium 151.964 |
64 Gd Gadolinium 157.25 |
65 Tb Terbium 158.925 |
66 Dy Dysprosium 162.500 |
67 Ho Holmium 164.930 |
68 Er Erbium 167.259 |
69 Pr Thulium 168.934 |
70 Yb Ytterbium 173.054 |
71 Lu Lutetium 174.97 |
| ** |
89 Ac Actinium 227 |
90 Th Thorium 232.038 |
91 Pa Protactinium 231.036 |
92 U Uranium 238.029 |
93 Np Neptunium 237 |
94 Pu Plutonium 244 |
95 Am Americium 243 |
96 Cm Curium 247 |
97 Bk Berkelium 247 |
98 Cf Californium 251 |
99 Es Einsteinium 252 |
100 Fm Fermium 257 |
M
101 Md Mendelevium 258 |
102 Cm Nobelium 259 |
103 Lr Lawrencium 262 |

Alkali Metals
- Shiny
- Soft
- Highly reactive
- Low melting points
- Low densities (lower than other metals)
- Low electronegativity
- Low ionization energy
- Good conductors of heat and electricity
- Ductile - the physical property of the metal which means if we pull the metal it's going to stretch rather than break,
- Malleable - The ability of a substance, usually metal, to be deformed or molded into a different shape,
The alkali metals are all:
at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1.
They can all be cut easily with a knife due to their softness, exposing a shiny surface that tarnishes rapidly in air due to oxidation by atmospheric moisture and oxygen (and in the case of lithium, nitrogen).
All the alkali metals react with water, with the heavier alkali metals reacting more vigorously than the lighter ones.
Because of their high reactivity, they must be stored under oil to prevent reaction with air, and are found naturally only in salts and never as the free elements.
Caesium
The fifth alkali metal, is the most reactive of all the metals.
Alkaline Earth Metals
- Shiny
- Hard
- Silvery-white
- Somewhat reactive.
- Low melting points, but higher than that of alkali metals.
- Low electronegativity.
- Low ionization energy.
- Good conductors of heat and electricity.
- Ductile - the physical property of the metal which means if we pull the metal it's going to stretch rather than break.
- Malleable - The ability of a substance, usually metal, to be deformed or molded into a different shape.
The alkaline earth metals are all:
at standard temperature and pressure and readily lose their outermost two electrons to form cations with charge +2.
All the alkaline earth metals except beryllium also react with water to form strongly alkaline hydroxides and, thus, should be handled with great care.
The heavier alkaline earth metals react more vigorously than the lighter ones.
Transition Metals
- Usually hard and tough, at standard temperature and pressure.
- Higher melting and boiling points,
- Low electronegativity
- Low ionization energy.
- Low electron affinity.
- High conductors of heat and electricity.
- Ductile - the physical property of the metal which means if we pull the metal it's going to stretch rather than break.
- Highly Malleable - The ability of a substance, usually metal, to be deformed or molded into a different shape.
Transition metals are one which forms one or more stable ions which have incompletely filled orbitals. They:
Transition Metals are low reactive with water and oxygen, which explains why they resist corrosion.
Post Transition Metals
- Soft
- Brittle - hard but liable to break easily, opposite of Ductile and malleable
- Lower melting and boiling points, compared to transition metals
- Low electronegativity
- Low ionization energy.
- Low electron affinity.
- Poor conductors of heat and electricity.
Post Transition metals are all:
Aluminum is the only post-transition metal that is considered to be very reactive.
Noble gases
- As their name suggests, they are all gas at standard temperature and pressure, except for oganesson. There haven't been enough atoms produced of oganesson to know its phase for certain, but most scientists predict it will be liquid or solid.
- Relatively low density
- Higher Ionization energy
- Unknown electronegativity
The noble gases also known as the inert gases or rare gases, are group 18 on the periodic table, which is the column of elements on the far right side of the table. There are seven noble gas elements: helium, neon, argon, krypton, xenon, radon, and oganesson.
Noble gases are the least reactive chemical elements. They are nearly inert because the atoms have a full valence electron shell, with little tendency to accept or donate electrons to form chemical bonds.
Lanthanides
- Relatively soft metals
- High melting points and boiling points
- Very reactive
The lanthanide or lanthanoid series of chemical elements comprises elements numbers 57- 71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as rare-earth elements or rare-earth metals, they are:
Although the lanthanides are sometimes called rare earths, the elements are not particularly rare. However, they are difficult to separate from one another.
Actinides
- All are radioactive
- The metals tarnish readily in the air.
- Actinide metals tend to be fairly soft.
- Actinides are very dense metals with distinctive structures.
- They react with boiling water or dilute acid to release hydrogen gas.
The actinide or actinoid series encompasses of elements numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium.
All actinides are radioactive and release energy upon radioactive decay; naturally occurring uranium and thorium, and synthetically produced plutonium are the most abundant actinides on Earth.
Uranium is very important for nuclear energy production as when “enriched” into U-235 concentrations can be used as fuel for nuclear power plants and the nuclear reactors that run naval ships and submarines.
The first atomic bomb ever created, subsequently used to bomb Hiroshima, was made from highly-enriched uranium-235.
Hydrogen
Intro
State at standard pressure and pressure
The most common isotope of this element
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Hydrogen is a chemical element with the symbol H and atomic number 1, atomic mass 1.0079. It is the lightest element.
It is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.
It is a gas of diatomic molecules having the formula H₂.
It is colorless, odorless, tasteless, non-toxic, and highly combustible.
For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons.
Hydrogen is nonmetallic (except it becomes metallic at extremely high pressures), and readily forms a single covalent bond with most nonmetallic elements, forming compounds such as water and nearly all organic compounds.
1s1
2.2
Hydrogen plays a particularly important role in acid-base reactions because these reactions usually involve the exchange of protons between soluble molecules.
Helium
Intro
State at standard pressure and pressure
The most common isotope of this element
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Helium is the chemical element with the symbol He, atomic number 2, and atomic mass 4,0026.
It is the second lightest and second most abundant element in the observable universe, after hydrogen.
Its boiling point is the lowest among all the elements, and it does not have a melting point at standard pressure.
It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas.
The most common isotope of helium in the universe is helium-4, the vast majority of which was formed during the Big Bang.
Noble Gas.
1s2
Unknown
Its abundance is similar to this in both the Sun and Jupiter, due to the very high nuclear binding energy (per nucleon) of helium-4, with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. Large amounts of new helium are created by the nuclear fusion of hydrogen in stars.
Lithium
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Lithium is a chemical element with the symbol Li, atomic number 3, and an atomic mass of 6,941.

It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element.
Like all alkali metals, lithium is highly reactive and flammable and must be stored in a vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil
Alkali metal
1s22s1
0.98
When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride.
Beryllium
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Beryllium is the chemical element with the symbol Be, atomic number 4, and an atomic mass of 9,0122.

It is steel-gray, strong, lightweight, and brittle. It is a divalent element that occurs naturally only in combination with other elements to form minerals.
Notable gemstones high in beryllium include beryl (aquamarine, emerald) and chrysoberyl.
Alkaline earth metal
1s22s2
1.57
It is a relatively rare element in the universe, usually occurring as a product of the spallation of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into heavier elements. Beryllium constitutes about 0.0004 percent by mass of Earth's crust. The world's annual beryllium production of 220 tons is usually manufactured by extraction from the mineral beryl, a difficult process because beryllium bonds strongly to oxygen.
Boron
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Boron is the chemical element with the symbol B, atomic number 5, and an atomic mass of 10,811.
In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form, it is a brown powder.
Metalloid
1s22s22p1
2.04
As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.
Carbon
Intro
State at standard pressure and pressure
The most common isotope of this element
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Carbon is the chemical element with the symbol C, atomic number 6 and an atomic mass of 12,011.
It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds
Solid.
Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of about 5,730 years.
Non-metal
1s22s22p2
2.55
Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen.
Nitrogen
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Nitrogen is a chemical element with the symbol N, atomic number 7, and an atomic mass of 14,007.
Two atoms of the element bond to form N2, a colorless and odorless diatomic gas.
Non-metal
1s22s22p3
3.04
Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen.
Oxygen
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Oxygen is the chemical element with the symbol 8, atomic number 8, and an atomic mass of 15,999.
Two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O2.
A highly reactive nonmetal
1s22s22p4
3.44
All plants, animals, and fungi need oxygen for cellular respiration, which extracts energy by the reaction of oxygen with molecules derived from food and produces carbon dioxide as a waste product. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as do the major constituent inorganic compounds of animal shells, teeth, and bone. Most of the mass of living organisms is oxygen as a component of water, the major constituent of lifeforms.
Flourine
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Flourine is a chemical element with the symbol F, atomic number 9, and an atomic mass of 18,998.
It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases.
A highly reactive nonmetal
1s22s22p5
3.98
Owing to the expense of refining pure fluorine, most commercial applications use fluorine compounds, with about half of the mined fluorite used in steelmaking. The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides, or into cryolite, which plays a key role in aluminium refining. Molecules containing a carbon-fluorine bond often have very high chemical and thermal stability; their major uses are as refrigerants, electrical insulation and cookware, and PTFE (Teflon). Pharmaceuticals such as atorvastatin and fluoxetine contain C−F bonds. The fluoride ion from dissolved fluoride salts inhibits dental cavities, and so finds use in toothpaste and water fluoridation. Global fluorochemical sales amount to more than US$69 billion a year.
Neon
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Neon is the chemical element with the symbol Ne, atomic number 10, and atomic mass 20,179.
It is a colorless, odorless, inert monatomic gas.
Noble gas
1s22s22p6
Unknown
It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon, and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum.
Sodium
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Sodium is a chemical element with the symbol Na, atomic number 11, and an atomic mass of 22,990.
It is a soft, silvery-white, highly reactive metal.
Alkali-Metal
1s22s22p63s1
0.93
The free metal does not occur in nature and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans.
Magnesium
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Magnesium is a chemical element with the symbol Mg, atomic number 12, and an atomic mass of 24,305.
It is a shiny gray metal having a low density, low melting point, and high chemical reactivity. Like the other alkaline earth metals, it occurs naturally only in combination with other elements.
Alkaline-Earth-Metal
1s22s22p63s2
1.31
This element is the eleventh most abundant element by mass in the human body and is essential to all cells and some 300 enzymes. Magnesium ions interact with polyphosphate compounds such as ATP, DNA, and RNA. Hundreds of enzymes require magnesium ions to function. Magnesium compounds are used medicinally as common laxatives and antacids (such as milk of magnesia), and to stabilize abnormal nerve excitation or blood vessel spasm in such conditions as eclampsia.
Aluminium
Intro
State at standard pressure and pressure
The stable isotopes of this element
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Aluminium is the chemical element with the symbol Al, atomic number 13, and an atomic mass of 26,982.
Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic, and ductile.
It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe
Metal
1s22s22p63s23p1
1.61
The strong affinity towards oxygen leads to aluminium's common association with oxygen in nature in the form of oxides; for this reason, aluminium is found on Earth primarily in rocks in the crust, where it is the third most abundant element after oxygen and silicon, rather than in the mantle, and virtually never as the free metal. It is obtained industrially by mining bauxite rock, which is high in aluminium minerals.
Silicon
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Silicon is the chemical element with the symbol Si, atomic number 14, and an atomic mass of 28,086.
It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor.
Metalloid
1s22s22p63s23p2
1.90
The late 20th century to early 21st century has been described as the Silicon Age (also known as the Digital Age or Information Age) because of the large impact that elemental silicon has on the modern world economy. The small portion of very highly purified elemental silicon used in semiconductor electronics is essential to the transistors and integrated circuit chips used in most modern technology such as smartphones and other computers. In 2019, 32.4% of the semiconductor market segment was for networks and communications devices, and the semiconductors industry is projected to reach $726.73 billion by 2027.
Phosphorus
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Phosphorus is a chemical element with the symbol P, atomic number 15, and an atomic mass of 30,976.
Elemental phosphorus exists in two major forms, white phosphorus, and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth.
Non-metal
1s22s22p63s23p3
2.19
Phosphorus is an element essential to sustaining life largely through phosphates, compounds containing the phosphate ion, PO43-. Phosphates are a component of DNA, RNA, ATP, and phospholipids, complex compounds fundamental to cells. Elemental phosphorus was first isolated from human urine, and bone ash was an important early phosphate source. Phosphate mines contain fossils because phosphate is present in the fossilized deposits of animal remains and excreta. Low phosphate levels are an important limit to growth in a number of plant ecosystems. The vast majority of phosphorus compounds mined are consumed as fertilizers. Phosphate is needed to replace the phosphorus that plants remove from the soil, and its annual demand is rising nearly twice as fast as the growth of the human population. Other applications include organophosphorus compounds in detergents, pesticides, and nerve agents.
Sulphur
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Sulphur is a chemical element with the symbol S, atomic number 16, and an atomic mass of 32,065.
Elemental sulfur is a bright yellow, crystalline solid at room temperature.
Non-metal
1s22s22p63s23p4
2.58
Being abundant in its native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum. The greatest commercial use of the element is the production of sulfuric acid for sulfate and phosphate fertilizers, and other chemical processes. Sulfur is used in matches, insecticides, and fungicides. Many sulfur compounds are odoriferous, and the smells of odorized natural gas, skunk scent, grapefruit, and garlic are due to organosulfur compounds. Hydrogen sulfide gives the characteristic odor to rotting eggs and other biological processes.
Chlorine
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Chlorine is a chemical element with the symbol Cl, atomic number 17, and an atomic mass of 35,453.
Because of its great reactivity, all chlorine in the Earth's crust is in the form of ionic chloride compounds, which includes table salt. It is the second-most abundant halogen (after fluorine) and twenty-first-most abundant chemical element in Earth's crust
Chlorine is a yellow-green gas at room temperature.
Non-metal
1s22s22p63s23p5
3.16
Elemental chlorine is commercially produced from brine by electrolysis, predominantly in the chloralkali process. The high oxidizing potential of elemental chlorine led to the development of commercial bleaches and disinfectants, and a reagent for many processes in the chemical industry. Chlorine is used in the manufacture of a wide range of consumer products, about two-thirds of them organic chemicals such as polyvinyl chloride (PVC), many intermediates for the production of plastics, and other end products which do not contain the element. As a common disinfectant, elemental chlorine and chlorine-generating compounds are used more directly in swimming pools to keep them sanitary.
Argon
Intro
State at standard pressure and pressure
The stable isotopes of this element
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Argon is the chemical element with the symbol Cl, atomic number 18, and an atomic mass of 39,948.
Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv).
Gas
Nearly all of the argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas.
Noble gas
1s22s22p63s23p6
Unknown
Argon is extracted industrially by the fractional distillation of liquid air. Argon is mostly used as an inert shielding gas in welding and other high-temperature industrial processes where ordinarily unreactive substances become reactive; for example, an argon atmosphere is used in graphite electric furnaces to prevent the graphite from burning. Argon is also used in incandescent, fluorescent lighting, and other gas-discharge tubes. Argon makes a distinctive blue-green gas laser. Argon is also used in fluorescent glow starters.
Potassium
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Potassium is the chemical element with the symbol K, atomic number 19, and an atomic mass of 39,098.
It is a silvery-white metal that is soft enough to easily cut with a knife.
Alkali Metal
1s22s22p63s23p64s1
0.82
Potassium ions are vital for the functioning of all living cells. The transfer of potassium ions across nerve cell membranes is necessary for normal nerve transmission; potassium deficiency and excess can each result in numerous signs and symptoms, including an abnormal heart rhythm and various electrocardiographic abnormalities. Fresh fruits and vegetables are good dietary sources of potassium. The body responds to the influx of dietary potassium, which raises serum potassium levels, by shifting potassium from outside to inside cells and increasing potassium excretion by the kidneys.
Calcium
Intro
State at standard pressure and pressure
Metal, Metalloid or Non-metal
Electron Configuration
Electronegativity
Additional info
Calcium is the chemical element with the symbol Ca, atomic number 20, and atomic mass 40,078.
Calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air.
Alkali Earth Metal
1s22s22p63s23p64s2
1.00
Calcium is the most abundant metal and the fifth-most abundant element in the human body. As electrolytes, calcium ions (Ca2+) play a vital role in the physiological and biochemical processes of organisms and cells: in signal transduction pathways where they act as a second messenger; in neurotransmitter release from neurons; in a contraction of all muscle cell types; as cofactors in many enzymes; and in fertilization. Calcium ions outside cells are important for maintaining the potential difference across excitable cell membranes, protein synthesis, and bone formation.